DCI® What is Corrosion? – TB-0905

This Technical Bulletin 0905 explains chloride induced corrosion of steel in concrete.
Steel in Concrete
The main component of steel reinforcement is iron (Fe). Iron atoms are stable when they contain an equal number of electrons and protons, but also when either two or three electrons are lost leaving a positive charge of two or three on the atom. In concrete, iron can exist in any of these three states, Fe0, Fe+2 or Fe+3. Charged iron atoms (Fe+2 and Fe+3) are more active because they react with negatively charged molecules or compounds to form neutral compounds. In each of the active states, Fe+2 (ferrous) and Fe+3 (ferric), several reactions can take place. These reactions will lead to either a protective oxide barrier surrounding the reinforcing steel, or to corrosion of the rebar.
In the absence of chlorides, the protective oxide barrier is formed. Due to the high pH of concrete (approximately 12.5 to 13.5), Fe+2 becomes Fe+3 and reacts with oxygen. This oxide barrier (technically termed “passivating layer”) protects the rebar against deterioration. It is found in other environments with a similar pH. Reinforced and prestressed concrete structures are stable and long-lasting when the concrete does not contain detrimental salts.
The Problem
The presence of chloride ions causes corrosion of steel reinforcement. These ions come primarily from de-icing salts and marine environments. They are also introduced in smaller quantities from certain additives, mix water and aggregates. If the chlorides are mixed into the concrete, they come in contact with the steel immediately. If chlorides enter the concrete from the surface, they diffuse through the concrete to the steel. The rate of migration depends on the quality of the concrete.
The amount of chlorides necessary to initiate corrosion is 1 1/2 lbs/yd3 (0.9 kg/m3). A typical unprotected parking structure subject to de-icing salts, with concrete water/cement ratio of 0.45 and 1 1/2 in. (38 mm) of cover could have 5 lbs/yd3 (3 kg/m3) chlorides at the rebar in less than 15 years.
When the chloride ions reach the steel, some of them find imperfections in the passivating layer. These defects occur naturally in the oxide, leaving small unprotected points on the steel. At these points, the chlorides react with the iron in the rebar. The chloride ions form soluble complexes with Fe+2 and move away from the steel. The complexes react with oxygen (O2) to form iron oxides (rust), such as FeO, Fe3O4 and Fe2O3. More chlorides move to the defects as the new rust compounds diffuse away from the steel. The original passivating layer is destroyed and a new layer cannot form. The corrosion process now continues unabated.
The diffusion of the iron-chloride complex away from the steel and the formation of solid corrosion products with a fourfold volume increase create an expansive force. This force easily overcomes the relatively low tensile strength of the concrete. Surface staining, popouts, spalls, and general disruption result in complete failure of a reinforced concrete system.
An Electrochemical Process
Corrosion is an electrochemical process—electrons are transferred from one atom to another—and can be represented as an electrochemical cell (Figure 1). The iron atoms lose electrons and react to form rust. This is known as the anodic reaction. In concrete, the anodic reaction is:
2 Fe + 4 OH– g 2 Fe (OH)2 + 4 e–
Electrons released by the iron move through the steel reinforcement to a location where oxygen reacts with the electrons and water to form hydroxide ions. The reaction which uses the electrons is known as the cathodic reaction:
O2 + 4 e– + 2 H2O g 4 OH–
Figure 1
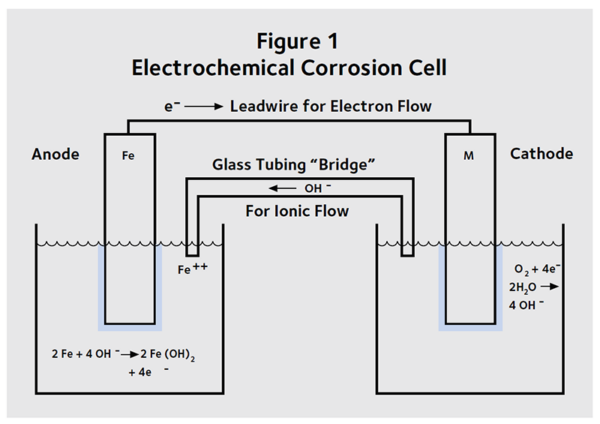
The OH– ions move through the concrete from the cathodic reaction to the anodic reaction, where they are consumed. The anodic and cathodic reactions are balanced.
Figure 2 shows the same electrochemical cell described in Figure 1 but as a concrete deck containing steel reinforcement. De-icing salts are spread on a bridge deck surface or brought into a parking garage with cars. The chloride ions diffuse through the concrete to the top mat of rebar, providing it with everything needed to become the anode. Oxygen can reach the bottom mat of rebar, and this steel provides a surface for the cathodic reaction. Electrons move through steel from the anode to the cathode, while OH– ions diffuse through the concrete from the cathode to the anode. This type of corrosion is known as macrocell corrosion.
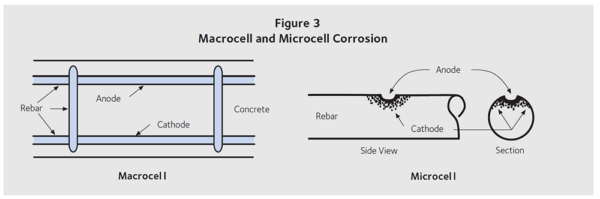
Anodic and cathodic reactions also occur on the same piece of steel. The reactions occur in the same way as described above, but the electrons and OH– ions move more easily from the anode to the cathode or vica versa. This type of corrosion is called microcell corrosion. Both types are shown in Figure 3.
Figure 2
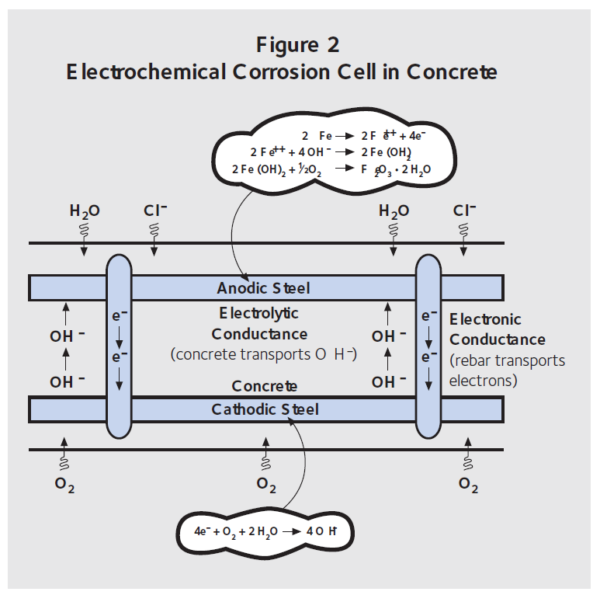
Figure 3
Summary
- In the absence of chlorides, concrete is able to protect steel reinforcement from corrosion, due to the alkaline environment.
- Corrosion is an electrochemical process. Chloride ions attack unprotected steel at defects in the protective oxide layer to start the corrosion process.
- Marine environments and de-icing salts are the major sources of chloride ions in concrete. Chlorides may also be found in the concrete components.
- The life of a reinforced concrete structure is shortened dramatically as a result of corrosion. If chloride ions are present, corrosion protection must be included in the structure





